Bionics scientific technologies takes pride of Photostability Test Chambers Manufacturers In India, In industries like pharmaceuticals, cosmetics and chemicals, product quality and safety are inevitable and even small changes in light or temperature or humidity can affect how a product performs over time. Many drugs to cosmetic products and chemical formulations are responsive to light exposure which can lead to degradation, discoloration or loss of effectiveness.
- What is a Photostability Chamber?
- Importance of Photostability Testing
- ICH Q1B Photostability Guidelines: Complete Overview and Requirements
- Stability Chambers in Pharmaceutical Applications
- How a Photostability Test Chamber Works
- Choosing the Right Stability Chamber Suppliers
- Applications of Photostability Chambers
- Photostability Test Chamber Video
- Advantages of Using Photostability Chambers
- Frequently Asked Questions : FAQs
- Q1. What is a photostability chamber and why is it important in pharmaceuticals?
- Q2.How does ICH Q1B recommend testing products for light stability?
- Q3. How does a stability chamber control temperature and humidity?
- Q4. What is the difference between photostability and ICH photostability testing?
- Q5. Can stability chambers be used in industries other than the pharmaceutical industry?
- Q6. What types of stability chambers are available?
- Q7. How do I choose reliable stability chamber suppliers?
- Q8. What is the role of a photostability test chamber in regulatory compliance?
- Q9. Are benchtop stability chambers effective for research labs?
- Q10. Why are stability cabinets preferred for bulk testing?
- Q11. What is the purpose of a photo stability chambers ?
- Q12. What is meant by 1.2 Million LUX hour for photostability
- Conclusion
Thats where photostability test chambers come into the picture because these specialized chambers are designed to affect light conditions both UV and visible to test how much life does a product have during storage or transport.These photostability test chambers are used widely in pharmaceutical stability testing, photostability chambers help companies meet strict standards like ICH Q1B that makes sure their products stay safe, effective and compliant from production to end use.
This guide covers everything that a person needs to know about photostability chambers what they are, how they work, why they matter in pharmaceutical stability testing and how they help ensure regulatory compliance and product safety.
What is a Photostability Chamber?
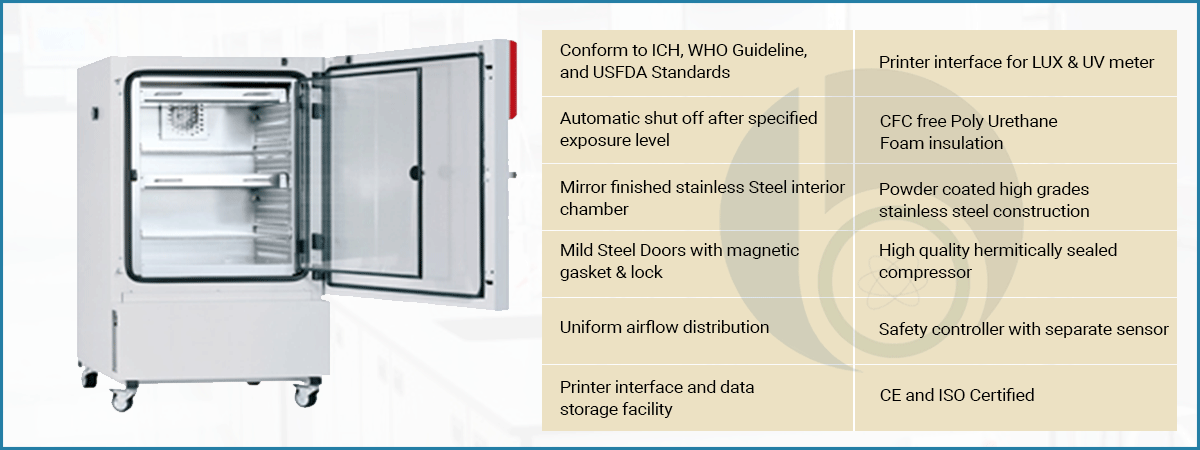
A photostability chamber is a machine used to test how light exposure affects drugs and chemicals. It is critical to maintain product safety and to meet compliance with global quality standards. Photostability chamber is a special kind of device designed to test medicines, cosmetics and paints react when exposed to light. This basically done for knowing it reaction when they come into sunlight and ultraviolet radiation. It combines temperature and humidity control with specialized light sources like UV and fluorescent lamps to recreate real world conditions.
Basically, it for testing purpose for manufacturer of these products, before going into production they first test their products by creating the environment, it help them to know whether their products are losing color or loose effectiveness, or degrade when left out in the light for a while. just like how a book’s pages can turn yellow or a cream might lose potency on a sunny windowsill. We at Bionics Scientific understand the importance of this process so we manufacture photo stability chambers according to ICH Guidelines.
Is your pharmaceutical testing truly meeting ICH Q1B guidelines?
Get a free quote for your ideal Photostability Chamber today. Connect us today at +91 9111161955 | 9376651333 | info@bionicsscientific.com
Importance of Photostability Testing
Photostability testing plays a major role in product development and quality assurance:
- Ensures Product Safety: Helps make sure medicines and chemicals stay safe and effective for long term use.
- Compliance with Global Regulations: Keeps in line with ICH guidelines as well as FDA, EMA and GMP standards.
- Shelf Life Accuracy: Supports manufacturers in setting the right expiration dates so products last as long as they should.
- Market approvals: Regulatory authorities demand stability data before product launches.
Without these tests the products might degrade which leads to health risks and financial losses.

ICH Q1B Photostability Guidelines: Complete Overview and Requirements
The ICH Q1B guidelines created by the International Council for Harmonisation to set global standards for testing how medicines react when exposed to light. Simply putting all together these guidelines help make sure that the drugs we rely on stay safe and effective even after being exposed to light during storage or use.
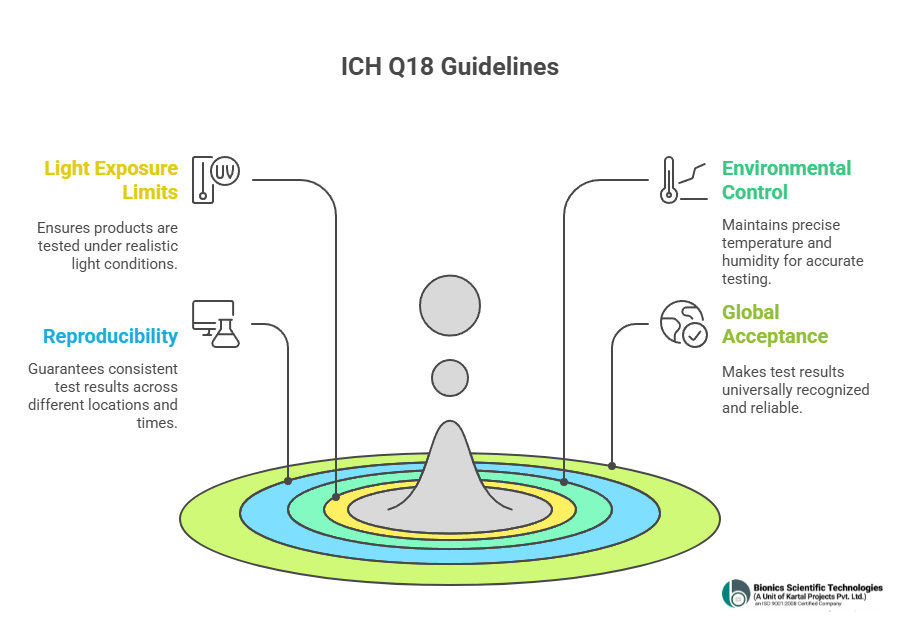
Following are the key points that covers ICH Q18:
- Light Exposure Limits: They basically tell how much UV and visible light a product needs to be tested with just like the kind of light it would actually face out in the real world.
- Environmental Control: The testing has to happen in carefully controlled temperature and humidity so the results are spot on and trustworthy.
- Reproducibility: These guidelines make sure that no matter where or when the test is done, it’s done the same way every time.
- Global Acceptance: Following these rules guidelines makes the result world wide acceptable and this avoids repeating of test again and again also make test more trustworthy and Reliable.
By following these widely accepted guidelines the pharmaceutical companies can feel good knowing they are testing their products the right way, keeping things compliant and most importantly making sure the medicines people rely on stay safe and effective.
Stability Chambers in Pharmaceutical Applications
Besides photostability chambers there are several types of stability chambers in pharmaceutical testing are widely used:
- Humidity and temperature stability chambers : Test products under controlled moisture and heat conditions.
- Binder stability chambers : Reliable chambers often used in pharmaceuticals for precision testing.
- Benchtop stability chambers : Compact units designed for R and D labs with limited space.
- Stability cabinets : Large storage systems for bulk testing and long term stability studies.
Each chamber type supports product testing under different environmental conditions also ensuring durability and compliance.
Talk to our experts for a custom built stability test chamber
We design, build and install stability test chambers for pharmaceutical use (Drug and Medicine Tests). These Photostability chambers are specifically designed to perform near UV and visual light testing with fluorescent lamps per ICH Q1B Guidelines, Option 2. Designed matching with international standards ICH, WHO Guideline, and USFDA Standards Contact us for specs & pricing: +91 9111161955 | 9376651333 | info@bionicsscientific.com
How a Photostability Test Chamber Works
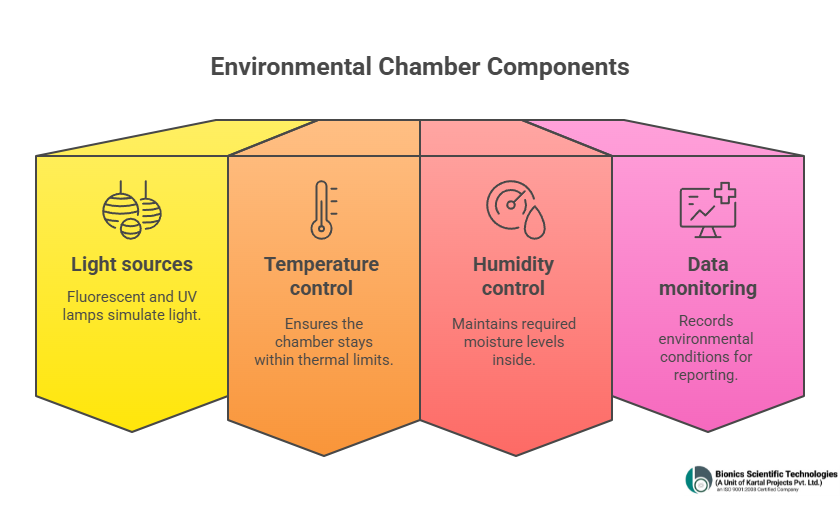
A photostability test chamber is engineered with advanced systems to replicate environmental exposure:
- Light sources : Fluorescent and UV lamps simulate both artificial and natural light.
- Temperature control : Ensures the chamber stays within precise thermal limits.
- Humidity control : Maintains required moisture levels.
- Data monitoring : Records environmental conditions for accurate reporting.
The precise control of stability chamber temperature and humidity ensures that test results are reliable and compliant with international guidelines.
Upgrade your stability testing with advanced Bionics chambers.
Get a world-class Photostability Test Chamber from Bionics Scientific, a leading Indian manufacturer. Contact Us for Specs & Pricing: +91 9111161955 | 9376651333 | info@bionicsscientific.com
Choosing the Right Stability Chamber Suppliers
Finding the right stability chamber suppliers is critical. Following points should be consider before purchase:
- Does the chamber comply with ICH guidelines for stability chambers?
- Is the equipment reliable and durable?
- Does the supplier provide after sales service and calibration?
- Can the chamber be customized according to size, capacity and features?
- Does the supplier have relevant experience in catering pharmaceutical and industrial clients?
Choosing reliable suppliers helps in making sure product testing is accurate and operations run smoothly. It is a simple step that supports long term success and peace of mind.
Applications of Photostability Chambers
Photostability test chambers are widely used across industries:
- Pharmaceutical Stability Testing: Ensuring Tablets, Capsules, Syrups and Injectables Stay Effective.
- Cosmetic Photostability Testing: How Creams, Perfumes and Skincare Products React to Light
- Food and beverages : Ensures packaging and consumables remain stable.
- Chemicals : Studying industrial and specialty chemical stability.
This makes photostability chambers a must have for any industry where product quality and compliance are critical.
Photostability Test Chamber Video
Advantages of Using Photostability Chambers
- Accurate results : Thanks to advanced temperature and humidity control.
- Global compliance : Fully meets ICH photostability requirements.
- Cost efficiency : Prevents recalls and customer complaints.
- Customer trust : Ensures safe and stable products reach to consumers.
This shift ensures compliance, saves time and supports innovation in pharmaceuticals and cosmetics.
Facing inconsistent results in your drug stability tests?
Get a world class Photostability Test Chamber from Bionics Scientific, a leading Indian laboratory products manufacturer. Contact Us for Specs & Pricing: +91 9111161955 | 9376651333 | info@bionicsscientific.com
Frequently Asked Questions : FAQs
Q1. What is a photostability chamber and why is it important in pharmaceuticals?

A photostability chamber is a machine used to test how light exposure affects drugs and chemicals. It is critical to maintain product safety and to meet compliance with global quality standards. Photostability chamber is a special kind of device designed to test medicines, cosmetics and paints react when exposed to light. This basically done for knowing it reaction when they come into sunlight and ultraviolet radiation.
Q2.How does ICH Q1B recommend testing products for light stability?

The ICH Q1B guidelines set international standards for photostability testing and defining light exposure and temperature requirements to ensure reliable results. ICH Q1B guidelines for photostability chambers require using specific light sources, like xenon arc lamps or fluorescent lamps, to simulate natural light and ensuring the light and UV exposure levels reach at least 1.2 million lux hours and 200 Watt hours/m² respectively, with proper monitoring and control of temperature, humidity, and homogeneous light distribution.
Q3. How does a stability chamber control temperature and humidity?
A stability chamber uses a combination of advanced sensors, heating cooling systems and humidifiers to precisely control temperature and humidity levels. These systems work together to maintain stable and consistent environmental conditions that ensures reliable results during stability testing.
Q4. What is the difference between photostability and ICH photostability testing?
Photostability testing checks how products react to light. ICH photostability testing follows specific international standards also known as ICH Q1B for regulatory approval.
Q5. Can stability chambers be used in industries other than the pharmaceutical industry?
Yes, stability chambers in pharmaceutical testing are also used in cosmetics also food packaging and chemical research for durability studies.
Q6. What types of stability chambers are available?
Options include humidity and temperature stability chambers, binder stability chambers, benchtop stability chambers and stability cabinets for large scale testing, Humidity Temperature Test Chamber
Q7. How do I choose reliable stability chamber suppliers?
Look for stability chamber suppliers that provide ICH compliant equipment, strong service support and customization options for specific needs. Like Bionics Scientific Technologies do from last 20 years.
Q8. What is the role of a photostability test chamber in regulatory compliance?

A photostability test chamber ensures compliance with ICH Q1B guidance, GMP and FDA requirements which are mandatory for global drug approval. Photostability chambers are used to control lightning, internal temperatures and sometimes humidity that follow strict standards to generate reliable data. These validated chambers environment ensures that the testing environment is as per the required regulatory requirements. So the test results are accurate and can be accepted by worldwide authorities. But in case we couldn’t provide such controlled testing, there is a chance of degradation, label inaccuracy, or even product rejection in the market due to non-compliance.
Q9. Are benchtop stability chambers effective for research labs?

Yes, benchtop stability chambers are ideal for small labs, offering precise control in a compact design, and perfect for R&D testing. In modern labs, researchers use the benchtop photostability chambers for different purposes, monitoring the durability of drugs and chemicals, testing the effects of environmental conditions on biological samples, and calibrating sensitive instruments. They deliver accurate and non-repeated results and an easy-to-use interface that saves a lot of time and operational costs. Their modular designs enable labs to scale their testing capacity as per the needs, no need to make heaviour investments in floor spaces, and bring larger equipment
Q10. Why are stability cabinets preferred for bulk testing?
Stability cabinets allow large scale storage and long term testing of pharmaceutical products which makes them essential for manufacturing plants.
Q11. What is the purpose of a photo stability chambers ?
The primary purpose of a photostability chamber is to test how products react to light presence, especially when it comes to UV Rays and Visible light, under controlled conditions. This is needed when identifying pharmaceuticals, cosmetics, food items, or other sensitive materials. It helps to diagnose whether the products lose potency, experience a change in color, or experience any other adverse effects when exposed to sunlight or artificial lighting during storage, or when kept in sunlight and used.
Q12. What is meant by 1.2 Million LUX hour for photostability
In photostability testing, the term 1.2 million LUX hours refers to the total amount of visible light exposure that a sample (such as a drug or formulation) receives during the test.
1.2 million LUX hours is the minimum required visible light dose during photostability testing to prove a product’s stability when exposed to light.
Conclusion
A photostability chamber is more than just a testing device but it is a safeguard for quality, compliance and safety. By following ICH Q1B guidelines and using reliable stability chamber suppliers the industries can ensure their products remain effective and safe worldwide.



