pH Meters, From labs to farms, Factories to kitchen – pH drives accuracy & Quality everywhere making it an indispensable and ubiquitous tool for safety check. In analytical testing a pH meter is a fundamental instrument designed to determine acidity or alkalinity of a solution having consistent pH results across water, soil & industrial applications.
Curious about how a pH meter works? In this blog, we’ll cover its principle, types, working diagram and real life uses – plus a simple guide, comparison chart and FAQs to make it easy for you.
Designed for accuracy , a pH meter measures the hydrogen ion concentration of solutions, indicating their acidity or alkalinity on a scale ranging from 0 to 14.
“A pH of 7 is neutral, with values below indicating acidity and above showing alkalinity. A pH meter consisting of a probe and a digital display, is widely used in laboratories, agriculture , water treatment, food production, and chemical industries. Accurate pH measurement is crucial for Quality control, chemical reactions, and environmental monitoring , making it an essential analytical tool.
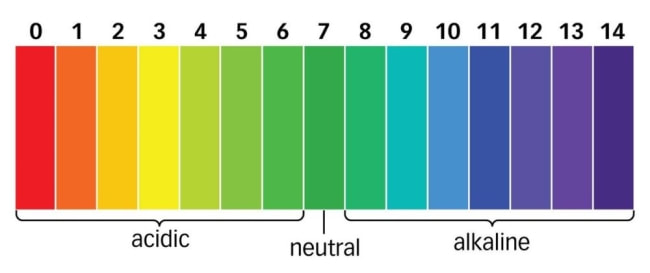
The pH scale shows how acidic or alkaline a solution is (0-14):
- pH<7 : Acidic
- pH = 7 : Neutral
- pH>7: Basic (Alkaline)
Display options:
- Digital screen (Modern Models)
- Analog Output (Traditional models)
Working Principle of a pH meter
- The principle is based on electrochemical potential difference.
- It measures the Voltage between two electrodes: the glass electrode (measuring electrode) and the reference electrode.
- When placed in a solution the glass electrode develops a potential that varies with the pH of the solution .
- The reference electrode maintains a constant potential , allowing accurate comparison.
How a pH meter Processes Reading
- The pH meter amplifies the potential difference between electrodes.
- It then converts this voltage into pH units displayed on the screen.
Formula used :
E = E₀ + (2.303 × RT/nF) × log [H⁺]
Where :
● E = potential measured
● E0 = Standard electrode potential
● R = Gas constant
● T = Temperature (in Kelvin)
● F = Faraday constant
● n = Charge number(usually 1 for H+)
● ⦗H+⦘ = Hydrogen ion concentration
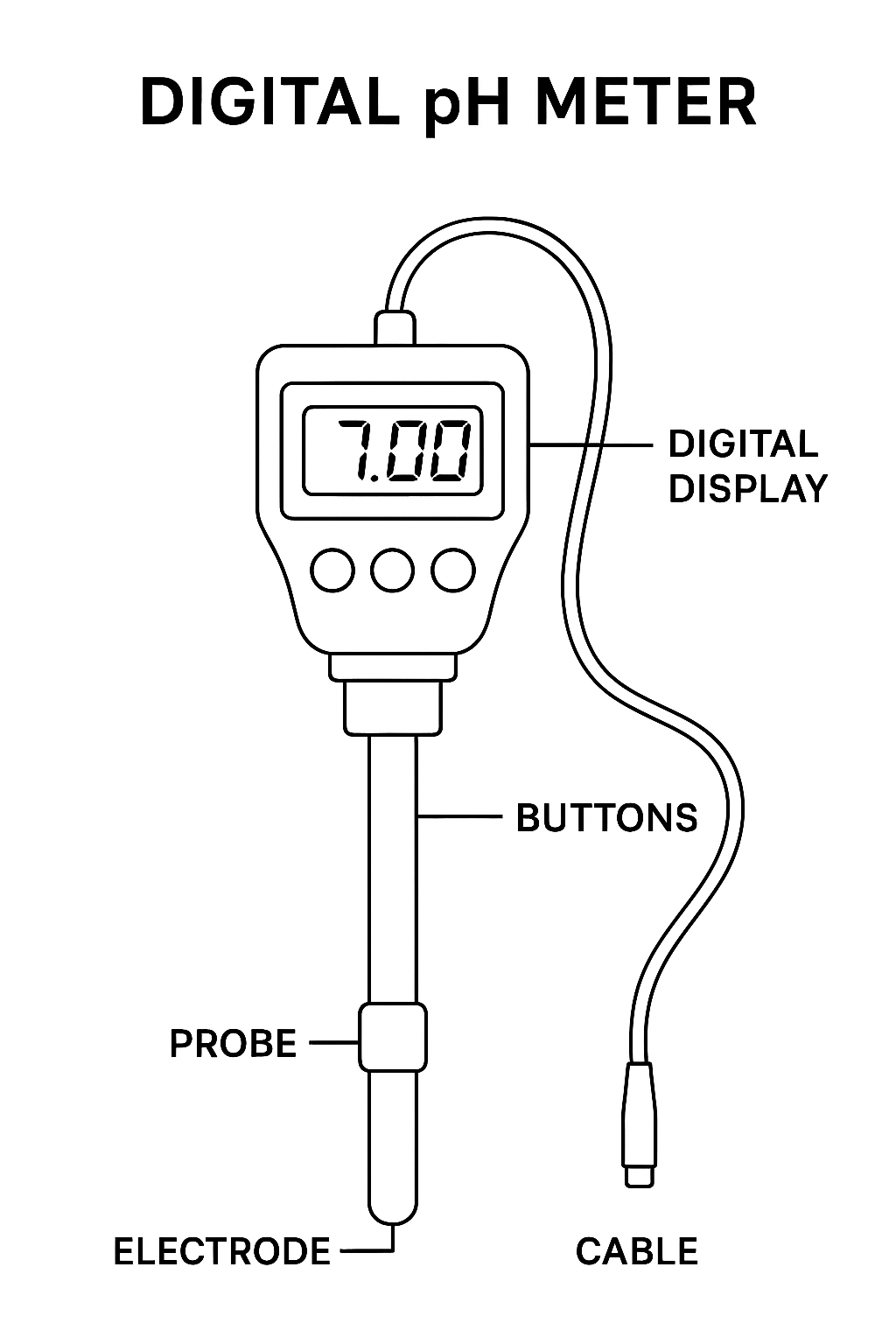
A pH meter has different parts that work together to give accurate readings:
- Glass Electrode → Feels how acidic or alkaline the liquid is.
- Reference Electrode → Keeps a steady point of comparison
- Temperature sensor → Fixes errors caused by temperature changes.
- Display Screen → Shows the pH value (digital or analog)
- Amplifier → Boosts the tiny electrical signal from the electrodes.
- Calibration Buttons / knobs → Help set the meter correctly before use.
Various types of pH meters are designed for specific applications:
- Digital pH meter – Provides accurate digital readouts, often with automatic calibration and temperature compensation. Commonly used in laboratories, industries and field testing.
- Portable pH Meter – Compact, battery operated device suitable for on-site testing in agriculture, wastewater management, etc.
- Pen- type pH Meter – Lightweight and small, ideal for aquariums, swimming pools, and quick sampling.
- Benchtop pH meter – High – precision instrument primarily used in research laboratories and quality control departments.
- pH Transmitters – Industrial grade devices that continuously measure and transmit pH values to control systems.
Choosing the right pH meter
| Type | How accurate? | Portability | Best for | Typical Cost |
| Digital | High | Medium | Labs, foods, beverage testing | ₹5,000 – 50,000 |
| Handheld | Moderate | Easy to carry | Farms, field checks | ₹2,000-15,000 |
| Pen-Type | Basic-Good | Very handy | Pools, aquariums, home use | ₹1,000 – 5,000 |
| Benchtop | Very precise | Not portable | Pharma & research labs | ₹15,000 – 1,00,000 |
| Industrial | Very precise | Rigid | Water plants, process industries | ₹20,000 – 1,20,000 |
India’s Most Reliable pH Meter Manufacturers
Bionics Scientific is a renowned manufacturer of pH meters, offering a wide range of models including modern microprocessor based instruments with automatic temperature adjustment. These meters are widely used in research organizations for precise and reliable measurement.
From advanced lab models to portable pen meters – Bionics Scientific covers it all. It offers high-quality pH meters for both national and international markets. Their microprocessor based models are an ideal choice for high-end pH measurements needs. The portable pH meters are scientifically designed to deliver maximum stability and quick results in field applications. In addition, Waterproof pen-type handheld pH meters are also available for convenient ,on the go-testing.
“Versatile pH meters trusted across agriculture, industry and water treatment.” Our pH meters are designed to meet the diverse needs and budget of customers. These instruments are highly useful across various sectors including agricultural and soil analysis laboratories, swimming pools, water quality monitoring in boiled feed water, water works department, fertilizers plants, petroleum refineries, breweries and water purification plants.
pH Scale Indicators:
Source: https://www.epa.gov/goldkingmine/what-ph
Bionics Scientific pH Meters
How pH Meters Make a Difference in Real Life?
The pH meter isn’t just for scientists- it’s useful in many parts of daily life:
- Science & Research – Provide aid to scientists for test reactions , study chemicals and even develop new medicines.
- Farming & Gardening – analyse if soil is too acidic or basic and helping plants grow healthier
- Food & drinks – Used to keep Yoghurt tangy, cheese tasty, and beer or wine just right
- Water quality – Makes sure water in pools, taps or factories is safe and balanced.
- Health & Medicine – Doctors test blood and urine pH to spot possible health problems early.
- Industry – Factories use it to make soaps, cosmetics, paper and other products with the right balance.
Inside a pH meter: A simple visual guide
A pH meter looks simple but powerful. A slim probe sits inside a beaker of liquid. At its tip is the glass bulb electrode, protected by a sleeve, next to the reference electrode. Wires connect the probe to a small digital meter that lights up with the pH Value for example,5.3 for orange juice. Sometimes a temperature probe is added to keep readings accurate. The setup shows clearly how the probe senses ions and the meter turns that signal into an easy-to-read number.
Source: https://en.wikipedia.org/wiki/PH_meter
The Secret behind pH meters: Turning signals into numbers
A pH meter works by comparing two tiny voltages. The glass bulb at the probe’s tip reacts with hydrogen ions in the liquid while the reference electrode stays steady. The difference between them changes with the acidity. At room temperature, each pH step equals about 59 millivolts. The meter boosts this weak signal and turns it into a pH number on the screen-after being set against known standards like pH 4 and pH 7 solutions.
Decoding pH Meter: Full form and Meaning
The term pH stands for Potential of Hydrogen(sometimes called power of Hydrogen). It simply measures how active hydrogen ions are in a solution. A pH meter is nothing mysterious – it’s just an instrument that reads this potential showing how acidic or basic a liquid is. The name is straightforward, rooted in the basic language of chemistry.
“Bionics Scientific Technologies: Why We’re the Preferred Choice”
Reliable pH meters , backed by experience and worldwide trust
When choosing a pH meter, the brand plays a critical role. Bionics scientific Technologies stands out due to:
- Top Notch Quality – Over 35 years of experience in manufacturing testing instruments, including pH meters , Recipient of a National Award For Quality from the Indian Government.
- Reliable accuracy – Precision instruments trusted by leading companies such as Amazon, Honda and Maruti for water soil and food testing.
- Excellent Support – Comprehensive after – sale service, user training , and guidance to ensure proper usage.
- Affordable options – A wide range of products from pocket meters to advanced lab models, catering to various budgets.
- Global Reach – Exporting to 50+ counties, meeting international standards for reliability and performance.
Choosing Bionics means picking a company that’s all about quality, trust, and making your work easier. Whether you’re a beginner or a pro, they’ve got your back!
What is the pH Scale?
The pH scale measures how acidic or alkaline a substance is. The scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic, and a pH greater than 7 is basic. Pure water is neutral, with a pH of 7.0. When chemicals are mixed with water, the mixture can become some level of either acidic or alkaline. Vinegar and lemon juice are acidic substances, while laundry detergents and ammonia are alkaline.
What is pH?
Acidic and alkaine (or “basic”) are two extremes that describe substances, usually liquids or chemicals, just like hot and cold are two extremes that describe temperature. A substance that is neither acidic nor alkaline is neutral.
Source: https://www.epa.gov/goldkingmine/what-ph